What is Sodium Saccharin
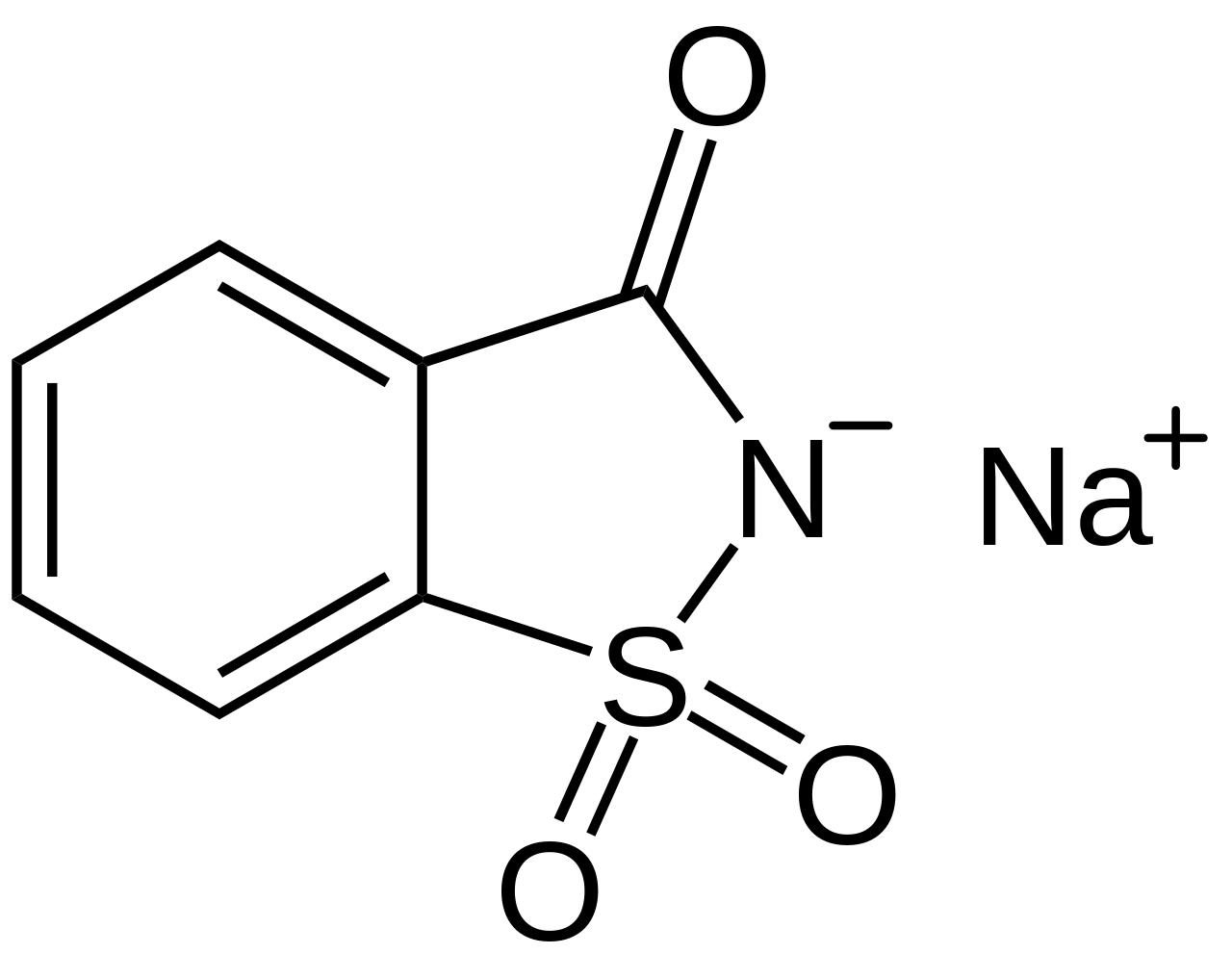
Sodium saccharin, or sodium ortho-sulphobenzimide with molecular formula C7H4NNaO3S, is the salt form of saccharin, an artificial sweetener. It is available in anhydrous and di-hydrated form. It is an odorless, white powder. It is 300 to 500 times sweeter than sugar (sucrose). Major application of Sodium saccharin is the food industry as an additive in different products. It is used as a low calorie sweetener and stabilizer in a variety of food and drinks. In bakeries it is used to sweeten baked goods, breads, cookies and muffins. Due to it’s rapidly dissolving nature in water it is used as an artificial sweetener in carbonated beverages and sodas.
Sodium saccharin is the solid form of the artificial sweetener saccharin. Saccharin is non-nutritive and is used to add sweetness to beverages and foods without the calories or detrimental effects of consuming sugar. Using artificial sweeteners can help you reduce your consumption of sugar. High sugar consumption is common and can contribute to a wide range of health concerns including Type 2 diabetes, obesity and cardiovascular disease.
Manufacturing Process
There are two main processes for making saccharin: First one is the Remsen-Fahlberg process and the second one is the Maumee, or Sherwin-Williams process, named upon the organization that further modified the Maumee process.
Remsen-Fahlberg Process
This process was named after the two scientists who discovered the sodium saccharine. In this process toluene is synthesized with chlorosulfonic acid to produce ortho and para-toluenesulfonyl chloride. Subsequent treatment with a potassium permanganate and ammonia forms the corresponding toluene sulfonamides.
Maumee Process
In early 1950, a new process for saccharin production was developed by USA-based Maumee Chemical Company. Earlier saccharin is produced from anthracitic acid by continuous production process. After the merger of Maumee Chemical Company and PMC Specialties group, the process was improved to a batch process by using purified methyl anthranilate, as a starting material which naturally occurs in grapes.
The Uses of Sodium Saccharin
In food industry it is used as non nutritive sweetener and stabilizer in a variety of food and cold drinks. In bakery industries it is used to sweeten baked goods, breads, cookies and muffins. In artificially sweetened diet drinks and sodas it is used because it is rapidly dissolved in water. Marzipan, plain, sweetened and fruit-flavored yogurt, jams/jellies and ice creams are the products that contain sodium saccharin.
Detergent Industry
Sodium saccharin is added to toothpastes and mouthwashes as a sweetening agent. Without adding sodium saccharin, toothpaste would have a bitter taste.
In cosmetics and personal care products, Sodium Saccharin is used in the formulation of dental products, mouthwashes and lipstick as sweetener and flavoring agent.
Other Applications
Sodium saccharin is used as an intermediate chemical ingredient in the Herbicides and pesticide manufacturing. It is used as a catalytic agent in the manufacture of anaerobic adhesives an adhesive that stiffens without existence the oxygen. They are commonly known as sealants or locking compounds which are used to seal closed or secure fitting parts. Sodium Saccharin is used in pharmaceutical industry as a coating on drugs.
Side Effects of Sodium Saccharin
According to a 1997 report written by the Center for the Science in Public Interest, or CSPI, saccharin sodium may act as a possible carcinogen and needs to be further investigated for its potential effect on an increase in the incidence of cancer. Although there has been no clear connection made between saccharin sodium and cancer in humans, it may act as a carcinogen, or cancer-causing compound, in rats and mice. Further research in this field must be conducted to determine the effect of saccharin sodium on cancer cells, particularly in relation to bladder cancer.
Weight Gain
Even though saccharin is an artificial sweetener that has no caloric value, the “Los Angeles Times” cited evidence that saccharin, and other artificial sweeteners, may increase the risk of obesity. The sweet taste of saccharin signals your body that it is about to receive a high amount of calories, and your digestive system prepares for the additional calories. When these calories do not come, your body may become resistant to this response, which can promote fat storage and weight gain.
Saccharin sodium may cause some individuals to experience a potentially severe allergic reaction, mainly due to the presence of sulfonamides. Symptoms of an allergic reaction to saccharin include headache, difficulty breathing, the sudden appearance of a skin rash or hives and diarrhea. In infants, an allergic reaction caused by saccharin used in some baby formulas may cause irritability and muscle dysfunction. If you believe you may be experiencing an allergic reaction to saccharin, seek medical attention immediately.
Diabetes
Saccharin sodium contains no caloric value and is not absorbed by your intestines. However, the sweet taste of saccharin may stimulate an endocrinological response, such as insulin production from your pancreas. The main effect of insulin is to transport sugar in the blood stream to various body tissues that can use it for energy. Without any sugar entering the blood stream after ingesting an artificial sweetener like saccharin sodium, insulin has nothing to bind on to. This may decrease your insulin sensitivity, which may increase your risk for developing diabetes.
PRODUCT IDENTIFICATION
| Common Name | Sodium Saccharin |
| CAS No. | 124-44-9 |
| Formula | C7H5NO3S.Na |
| Molecular Weight | 205.2 g mol-1 |
| H.S Code | 2925.11.00 |
| Synonyms | Saccharin sodium salt |
PHYSICAL AND CHEMICAL PROPERTIES
| Property | Units | Value |
|---|---|---|
| Appearance | White Crystalline Powder | |
| Purity | % | 99 (min) |
| Ammonium salt | ppm | 25 (max) |
| Arsenic | ppm | 3.0 (max) |
| Loss on dying | ppm | 15 (max) |
| Heavy metal | ppm | 10 (max) |
| P-toluene sulfonamide | ppm | 10 (max) |
| O-toluene sulfonamide | ppm | 10 (max) |
| Benzoic acid-sulfonamide | ppm | 25 (max) |
| Particle size | mesh | 8-12; 20-40; 40-80 |

